Package labeling and registration
This section of the website applies to requirements of the Technical regulations on cosmetic products that will become effective from August 3, 2022. For earlier requirements please refer to the following page.
Package of cosmetic products must contain the necessary information that meets the requirements of the Technical Regulations and other legislative acts before placing on the market. Each individual cosmetic product is subject to labeling.
Information should be visible, legible and durable, can be placed by applying a sticker, on a tab (leaflet), tag or card.
Labeling language – state (Ukrainian). Information in other languages may be placed next to the text in Ukrainian. The use of abbreviations or symbols is also permitted.
Mock-ups (graphics file and/or photograph) are subject to registration with the competent authority.
The Responsible Person is responsible for the conformity of the labeling with the requirements of the Technical Regulations. The distributor (every subject in the supply chain, other than the manufacturer or importer) is obliged to ensure that the required labeling information is available before the product is placed on the market.
|
Since 2006 we provide professional services of certification of cosmetic products in Ukraine. We have deep knowledge of Ukrainian and European legislation, experience, and necessary resources. Our services:
Our team has an excellent knowledge of the legislation, fluently communicates in English, possesses the broad experience, and will be able to answer all questions. Cooperation with us will grant you high-quality professional service and fast results. |
Information on the label
According to the requirements of the Law of Ukraine “On Ensuring the Functioning of the Ukrainian Language as the State Language”, the labeling language is Ukrainian. Other languages are allowed.
Labeling information includes, but is not limited to:
- name and address of the responsible person;
- country of origin for imported products;
- units of mass or volume, or quantity of units if mass or volume does not matter;
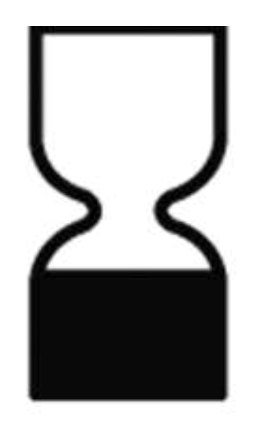
- the minimum expiration date and the symbol or phrase “use before”;
- storage conditions and/or conditions under which the specified expiration date is guaranteed;
- if necessary, a period of time after opening during which the product is safe and can be used without harm to the consumer (indicated by the symbol);
- precautions;
- features of products intended for professional use;
- manufacturing lot number (batch) or data for identification;
- the purpose of cosmetic products, if it is not obvious;
- list of ingredients (“ingredients” or “composition”) in decreasing order of their mass fraction, including nanomaterials and coloring materials, using the generally accepted International Nomenclature of Cosmetic Ingredients (INCI);
- name and address of the manufacturer;
- date of manufacture;
- if a product may be hazardous to the health or property, or to the environment, then information on such properties and possible consequences.
When using ingredients, coloring materials, preservatives and UV filters included in Appendices 3-6 of the Technical Regulations in the composition of a cosmetic product, appropriate conditions and warnings must be indicated on the labeling.
The labeling of cosmetic products must not contain information, trademarks, photographs or images that may give reason to believe that the product has characteristics or properties that it does not possess.
The label may contain information that the product and its ingredients have not been studied on animals.
Symbols for the labeling of cosmetic products
The technical regulation establishes graphic symbols, the use of which makes it possible not to duplicate information in different languages:
|
|
|
|
| See information in accompanying materials | The period after opening the package | Minimum expiration date |
Units of measurement
Units of measurement on the labeling of cosmetic products must be reflected using the International System of Units SI, in accordance with the requirements of Order of the Ministry of Economic Development dated August 5, 2015 No. 914.
Registration of mock-ups (graphics files)
When cosmetic products are introduced into circulation, the Responsible Person shall notify (register) the graphics file of the labeling and, if necessary, the photograph of the package. The competent authority responsible for the registration of graphics files is the Ministry of Health of Ukraine.
Our services
Cratia provides comprehensive regulatory support and accompanies at all stages of regulatory compliance of cosmetic products in Ukraine:
- outsourcing of a Responsible Person (RP) in Ukraine;
- assessment of the composition of the product;
- filling of the Product Information File;
- Cosmetic Product Safety Report (CPSR);
- verification/development of labeling, instructions, and advertising materials;
- product notification;
- development and implementation of Good Manufacturing Practice in accordance with ISO 22716 (GMP);
- legal and consulting support of the manufacturer, Responsible person or distributor (importer).
We possess deep knowledge and experience, speak and write fluent English. We will take over the organization of the process, assist in filling of the set of documents and perform the required procedures in a short time.
We provide preliminary consultations free of charge, call us by phone +38 (068) 064-78-31, +38 (044) 223-61-67, or write to info@cratia.ua, or come to the meeting directly to our office.